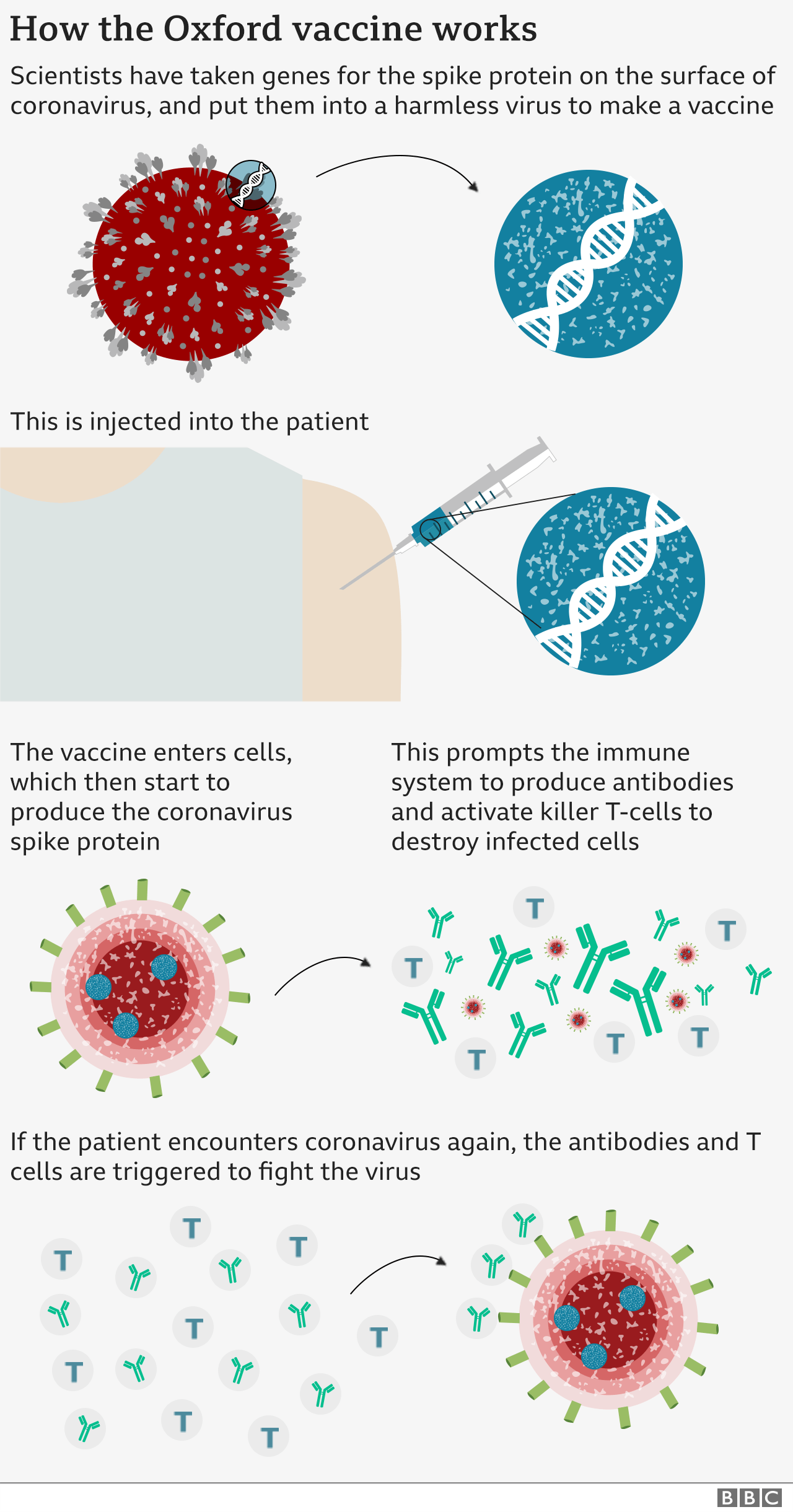
Derek Lowe, the pharma medicinal chemist whose blog I have often linked:
 blogs.sciencemag.org
blogs.sciencemag.org
Good info and very good hyperlinks.
The questions, and his bottom-line final conclusion (after a bit of analysis)
How long will the vaccine protection last?
...a strong, normal, lasting immune response in the great majority of patients. Add in the results we’re seeing from the two vaccines that have reported interim data so far, and I think that the prospects for lasting immunity from vaccination are also very good. Remember, the early vaccine data suggested antibody responses at least as strong as those found in naturally infected cases.
How effective are these vaccines? Will they provide total protection or not?
... the results we have so far indicate that these vaccines will indeed provide strong protection in the great majority of patients. The number of asymptomatic cases among the vaccinated population will be a harder number to pin down, but I believe that we should be in good enough shape there as well, based on antibody levels in the primate studies and what we’re seeing in humans.
What about coronavirus mutations? Will the virus move out from under the vaccine’s targeting?
...the coronavirus can’t undergo the wholesale changes that we see with the influenza viruses. And the mutations we’re seeing so far appear to still be under the umbrella of the antibody protection we’ll be raising with vaccination, which argues that it’s difficult to escape it.
What about efficacy in different groups of people? Where will the vaccines work the best, and where might there be gaps?
...too soon to tell, but our first look at efficacy in older patients is very good indeed, and that’s the most significant high-risk patient subgroup taken care of right off the top.
How safe are these vaccines? What do we know about side effects?
...immediate safety looks good so far. Rare side effects and long-term ones are still possible, but based on what we’ve seen with other vaccines, they do not look to be anywhere at all significant compared to the pandemic we have in front of us.
OK, what about the rollout? Who’s getting these things first? When does everyone else get a chance to line up?
Harder questions to answer... the very first people to get these new vaccines will almost surely be health care workers, and starting some time on in December. The rollout after that has too many variables to usefully predict, but it’s going to be the biggest thing of its type ever attempted, in people-per-unit-time. And yes, I think it’s going to work, and not a minute too soon.
AAAS
Good info and very good hyperlinks.
The questions, and his bottom-line final conclusion (after a bit of analysis)
How long will the vaccine protection last?
...a strong, normal, lasting immune response in the great majority of patients. Add in the results we’re seeing from the two vaccines that have reported interim data so far, and I think that the prospects for lasting immunity from vaccination are also very good. Remember, the early vaccine data suggested antibody responses at least as strong as those found in naturally infected cases.
How effective are these vaccines? Will they provide total protection or not?
... the results we have so far indicate that these vaccines will indeed provide strong protection in the great majority of patients. The number of asymptomatic cases among the vaccinated population will be a harder number to pin down, but I believe that we should be in good enough shape there as well, based on antibody levels in the primate studies and what we’re seeing in humans.
What about coronavirus mutations? Will the virus move out from under the vaccine’s targeting?
...the coronavirus can’t undergo the wholesale changes that we see with the influenza viruses. And the mutations we’re seeing so far appear to still be under the umbrella of the antibody protection we’ll be raising with vaccination, which argues that it’s difficult to escape it.
What about efficacy in different groups of people? Where will the vaccines work the best, and where might there be gaps?
...too soon to tell, but our first look at efficacy in older patients is very good indeed, and that’s the most significant high-risk patient subgroup taken care of right off the top.
How safe are these vaccines? What do we know about side effects?
...immediate safety looks good so far. Rare side effects and long-term ones are still possible, but based on what we’ve seen with other vaccines, they do not look to be anywhere at all significant compared to the pandemic we have in front of us.
OK, what about the rollout? Who’s getting these things first? When does everyone else get a chance to line up?
Harder questions to answer... the very first people to get these new vaccines will almost surely be health care workers, and starting some time on in December. The rollout after that has too many variables to usefully predict, but it’s going to be the biggest thing of its type ever attempted, in people-per-unit-time. And yes, I think it’s going to work, and not a minute too soon.