Arturo Casadevall and collaborators at Johns Hopkins and beyond have worked around the clock to develop a convalescent serum therapy to treat COVID-19 using blood plasma from recovered patients. If early promising studies on the therapy done in China are confirmed by U.S. trials, thousands of survivors might soon line up to donate their antibody-rich plasma. "I absolutely think this could be the best treatment we have for the next few months," Hopkins pathologist Aaron Tobian says.
https://hub.jhu.edu/2020/04/08/arturo-casadevall-blood-sera-profile/
Under the leadership of immunologist Arturo Casadevall, Johns Hopkins has spearheaded the use of a convalescent serum therapy, a potential COVID-19 treatment—with an old pedigree. On March 24, the U.S. Food and Drug Administration began allowing researchers to request emergency authorization for its use. Within three days hospitals in Houston and New York City started treatments, and now under a FDA "expanded access program" soon "a very large number" of U.S. hospitals will follow suit, according to Tobian.
On Friday, the FDA approved a clinical trial specifically for Johns Hopkins that will allow its researchers to further test the therapy as a means of preventing otherwise healthy people, notably front-line medical staff, from getting sick. FDA approval is pending for a second Hopkins clinical trial on patients who are slightly or moderately ill to see if the serum will keep them out of ICUs and help bring them back to health.
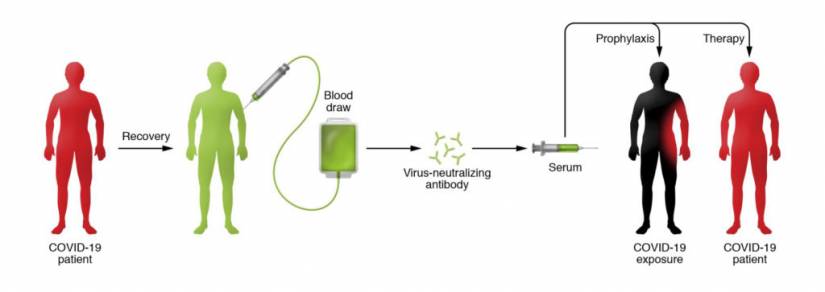
In recent weeks, Casadevall has led a team of physicians and scientists from around the United States to establish a network of at least 40 hospitals and blood banks in 20 states that can begin collecting, isolating, and processing blood plasma from COVID-19 survivors. People who recover from an infection develop antibodies that circulate in the blood and can neutralize the pathogen. Researchers hope to use the technique to treat critically ill COVID-19 patients and boost the immune systems of health care providers and first responders. Currently, there are no proven drug therapies or effective vaccines for treating the novel coronavirus.
"At the end of January, I knew this disease was going to get out of China, and I knew there was a huge history of the use of plasma and serum in the 20th century," says Casadevall, a Bloomberg Distinguished Professor of molecular microbiology and immunology and infectious diseases at the Johns Hopkins Bloomberg School of Public Health and School of Medicine. "This [medical effort] has become a juggernaut… We're racing to deploy this."
Thousands of survivors might soon line up to donate their antibody-rich plasma, according to physicians. But that's only if early promising studies on the therapy done in China are confirmed by U.S. trials that show "dramatic effects and improvements" in patients, according to Tobian. He is optimistic the therapy will do just that. "I absolutely think this could be the best treatment we have for the next few months."
This passive-antibody therapy has been used since the 1890s to combat diseases as wide-ranging as measles, SARS, Ebola, H1N1 flu, and polio—and holds the promise of keeping the virus at bay until a vaccine can be developed. (Current estimates are that a vaccine for emergency use could be available by early 2021.) During the SARS outbreak in 2002–2003, an 80-person trial of convalescent serum in Hong Kong found that people treated with it within two weeks of showing symptoms had a higher chance of being discharged from hospital than did those who weren't treated.
The beauty of the therapy, says Casadevall, is that it involves the well-established—and safe—method of blood donation. Except in this case, survivors' plasma (or serum), which contains the antibody to COVID-19, is separated from red blood cells and transfused into the three categories of recipients: the critically ill as a last-stop "compassionate care" measure; patients who are slightly or moderately ill to keep them out of ICUs and off scarce ventilators, and front-line medical workers to prevent them from getting sick. Nearly a cup of the serum (200 milliliters, or one unit) would be administered to each recipient, according to Tobian, with each donor providing enough serum for up to four patients. (Each donor, depending on body size, can provide two to four units.)
Casadevall had the vision for this treatment. He also had the wisdom not to micromanage his team of doctors, whom he set free to create their own fast-flowing mini-teams. Together, they united with peers around the world in a marathon of selfless, round-the-clock work toward an urgent common goal—to overwhelm and crush the COVID-19 virus. "Looking forward to another day of working with an incredible set of colleagues," tweeted Casadevall in late March. "Day began at 4 a.m. and will go to near midnight." In this process, doctors, researchers, and regulators from as far away as Israel and Ethiopia banded with Hopkins doctors to create treatment protocols, open labs, win regulatory and institutional approvals, identify donors, compile data, and organize and share vital information. The research effort received a welcome boost in late March with a gift of $3 million from Bloomberg Philanthropies and $1 million in funding from the state of Maryland.
"We usually spend a year preparing for the next flu season," says Andy Pekosz, vice chair of the Department of Molecular Microbiology and Immunology at the Bloomberg School of Public Health. "What we do for flu in a year, we're trying to do in a month for COVID-19. Our window of acting is a small one." The fast-spreading coronavirus has already killed at least 70,000 people around the world, with nearly 1,300,000 total confirmed cases. Numbers that continue to grow.
From the beginning, Casadevall knew he faced more than a medical problem. Plasma therapy's history was unknown to most people, so he needed to draw public attention to his cause. Realizing a medical journal commentary would reach a limited audience, Casadevall shopped around an editorial that urged the use of convalescent serum. The essay, published in the Feb. 27 edition of The Wall Street Journal, told the story of an ingenious Pottstown, Pennsylvania, doctor who in 1934 arrested a measles outbreak at a boys boarding school by using serum therapy. "A remarkable victory against a highly contagious disease," Casadevall wrote.

https://hub.jhu.edu/2020/04/08/arturo-casadevall-blood-sera-profile/
Under the leadership of immunologist Arturo Casadevall, Johns Hopkins has spearheaded the use of a convalescent serum therapy, a potential COVID-19 treatment—with an old pedigree. On March 24, the U.S. Food and Drug Administration began allowing researchers to request emergency authorization for its use. Within three days hospitals in Houston and New York City started treatments, and now under a FDA "expanded access program" soon "a very large number" of U.S. hospitals will follow suit, according to Tobian.
On Friday, the FDA approved a clinical trial specifically for Johns Hopkins that will allow its researchers to further test the therapy as a means of preventing otherwise healthy people, notably front-line medical staff, from getting sick. FDA approval is pending for a second Hopkins clinical trial on patients who are slightly or moderately ill to see if the serum will keep them out of ICUs and help bring them back to health.
In recent weeks, Casadevall has led a team of physicians and scientists from around the United States to establish a network of at least 40 hospitals and blood banks in 20 states that can begin collecting, isolating, and processing blood plasma from COVID-19 survivors. People who recover from an infection develop antibodies that circulate in the blood and can neutralize the pathogen. Researchers hope to use the technique to treat critically ill COVID-19 patients and boost the immune systems of health care providers and first responders. Currently, there are no proven drug therapies or effective vaccines for treating the novel coronavirus.
"At the end of January, I knew this disease was going to get out of China, and I knew there was a huge history of the use of plasma and serum in the 20th century," says Casadevall, a Bloomberg Distinguished Professor of molecular microbiology and immunology and infectious diseases at the Johns Hopkins Bloomberg School of Public Health and School of Medicine. "This [medical effort] has become a juggernaut… We're racing to deploy this."
Thousands of survivors might soon line up to donate their antibody-rich plasma, according to physicians. But that's only if early promising studies on the therapy done in China are confirmed by U.S. trials that show "dramatic effects and improvements" in patients, according to Tobian. He is optimistic the therapy will do just that. "I absolutely think this could be the best treatment we have for the next few months."

This passive-antibody therapy has been used since the 1890s to combat diseases as wide-ranging as measles, SARS, Ebola, H1N1 flu, and polio—and holds the promise of keeping the virus at bay until a vaccine can be developed. (Current estimates are that a vaccine for emergency use could be available by early 2021.) During the SARS outbreak in 2002–2003, an 80-person trial of convalescent serum in Hong Kong found that people treated with it within two weeks of showing symptoms had a higher chance of being discharged from hospital than did those who weren't treated.
The beauty of the therapy, says Casadevall, is that it involves the well-established—and safe—method of blood donation. Except in this case, survivors' plasma (or serum), which contains the antibody to COVID-19, is separated from red blood cells and transfused into the three categories of recipients: the critically ill as a last-stop "compassionate care" measure; patients who are slightly or moderately ill to keep them out of ICUs and off scarce ventilators, and front-line medical workers to prevent them from getting sick. Nearly a cup of the serum (200 milliliters, or one unit) would be administered to each recipient, according to Tobian, with each donor providing enough serum for up to four patients. (Each donor, depending on body size, can provide two to four units.)
Casadevall had the vision for this treatment. He also had the wisdom not to micromanage his team of doctors, whom he set free to create their own fast-flowing mini-teams. Together, they united with peers around the world in a marathon of selfless, round-the-clock work toward an urgent common goal—to overwhelm and crush the COVID-19 virus. "Looking forward to another day of working with an incredible set of colleagues," tweeted Casadevall in late March. "Day began at 4 a.m. and will go to near midnight." In this process, doctors, researchers, and regulators from as far away as Israel and Ethiopia banded with Hopkins doctors to create treatment protocols, open labs, win regulatory and institutional approvals, identify donors, compile data, and organize and share vital information. The research effort received a welcome boost in late March with a gift of $3 million from Bloomberg Philanthropies and $1 million in funding from the state of Maryland.
"We usually spend a year preparing for the next flu season," says Andy Pekosz, vice chair of the Department of Molecular Microbiology and Immunology at the Bloomberg School of Public Health. "What we do for flu in a year, we're trying to do in a month for COVID-19. Our window of acting is a small one." The fast-spreading coronavirus has already killed at least 70,000 people around the world, with nearly 1,300,000 total confirmed cases. Numbers that continue to grow.
From the beginning, Casadevall knew he faced more than a medical problem. Plasma therapy's history was unknown to most people, so he needed to draw public attention to his cause. Realizing a medical journal commentary would reach a limited audience, Casadevall shopped around an editorial that urged the use of convalescent serum. The essay, published in the Feb. 27 edition of The Wall Street Journal, told the story of an ingenious Pottstown, Pennsylvania, doctor who in 1934 arrested a measles outbreak at a boys boarding school by using serum therapy. "A remarkable victory against a highly contagious disease," Casadevall wrote.